Podcast: Embed
Subscribe: Apple Podcasts | Spotify | Amazon Music | Android | Pandora | iHeartRadio | Blubrry | TuneIn | Deezer | RSS
CardioNerds (Amit Goyal & Daniel Ambinder) join Scripps cardiology fellows (Christine Shen and Andrew Cheng) for some Cardiology and California Burritos in San Diego! They discuss an informative case of Wet Beriberi and Stiff Left Atrial Syndrome. Dr. Thomas Heywood provides the E-CPR and program director Dr. Malhar Patel provides a message for applicants. Episode notes were developed by Johns Hopkins internal medicine resident Tommy Das with mentorship from University of Maryland cardiology fellow Karan Desai.
Jump to: Patient summary – Case media – Case teaching – References

The CardioNerds Cardiology Case Reports series shines light on the hidden curriculum of medical storytelling. We learn together while discussing fascinating cases in this fun, engaging, and educational format. Each episode ends with an “Expert CardioNerd Perspectives & Review” (E-CPR) for a nuanced teaching from a content expert. We truly believe that hearing about a patient is the singular theme that unifies everyone at every level, from the student to the professor emeritus.
We are teaming up with the ACC FIT Section to use the #CNCR episodes to showcase CV education across the country in the era of virtual recruitment. As part of the recruitment series, each episode features fellows from a given program discussing and teaching about an interesting case as well as sharing what makes their hearts flutter about their fellowship training. The case discussion is followed by both an E-CPR segment and a message from the program director.
CardioNerds Case Reports Page
CardioNerds Episode Page
CardioNerds Academy
Subscribe to our newsletter- The Heartbeat
Support our educational mission by becoming a Patron!
Cardiology Programs Twitter Group created by Dr. Nosheen Reza

Patient Summary
A woman in her mid-60s with history of rheumatic mitral stenosis s/p mechanical mitral valve replacement, HFpEF, and paroxysmal atrial fibrillation s/p ablation presents with subacute worsening dyspnea despite escalating diuretic doses. TTE shows an EF of 62%, normal gradients across the mitral valve without mitral regurgitation, and a dilated IVC. She is admitted with a presumed diagnosis of decompensated heart failure, and started given IV furosemide. Her symptoms slightly improve though do not resolve, and her creatinine increases from 1.4 to 2.1.
In light of the unclear hemodynamic picture, a RHC is done, showing a RA pressure 9, RV pressure of 80/10, PAP 70/25 with mPAP 40, PCWP 30, SVR 872, CO 11 (by thermodilution), and CI 5.2. Notably, large V waves are noted on the RHC. Given concern for mitral regurgitation in the setting of large V waves, a TEE was pursued, which confirmed the lack of MR seen on TTE. Thus, her large V waves were felt to be due to stiff left atrial syndrome, and a cardiac CT showed a severely calcified “coconut left atrium”. Labwork revealed a profoundly low thiamine level (21, with LLN of 70), raising concern for wet beri beri syndrome.
The patient’s unifying diagnosis was indolent left atrial syndrome that was exacerbated by high outout heart failure due to Wet Beri Beri syndrome. The patient received thiamine supplementation, and was diuresed to euvolemia with dramatic improvement in symptoms. A repeat RHC after thiamine replacement showed a CO of 5.7 and CI of 2.74 by thermodilution, demonstrating resolution of her high output heart failure.
Case Media
A. CXR
B. ECG
C. RHC: large V waves are noted on the RHC
D. CO 11 and CI 5.2 by thermodilution pre-treatment
E. Cardiac CT showed a severely calcified “coconut left atrium”
F. Repeat CO of 5.7 and CI of 2.74 by thermodilution after thiamine replacement
Episode Schematics & Teaching
The CardioNerds 5! – 5 major takeaways from the #CNCR case
1) This case featured a patient with Stiff Left Atrial Syndrome! Cardionerds, what the heck is that?
- Stiff Left Atrial Syndrome (SLAS) is fundamentally a disorder of atrial compliance, wherein a non-compliant left atrium (LA) leads to abnormal atrial diastole. During LV systole (atrial diastole), the LA receives blood from the low-resistance pulmonary veins. Under normal conditions, the LA pressures initially fall (x-descent). Then, as the atrium fills from both RV contraction and passive filling from the pulmonary veins, there is a steady and modest rise in LA pressure (v-wave). In patients with decreased LA compliance, the V-wave may be accentuated.
- In SLAS, left atrial compliance is significantly decreased, leading to very large v-waves that reflect the inability to accommodate LA filling and the steepened slope of the pressure-volume curve (see the below diagram from Urey et al). This leads to dramatically increased LA pressures during LV systole (especially in late LV systole), contributing to post-capillary pulmonary hypertension over time and symptoms of dyspnea on exertion.
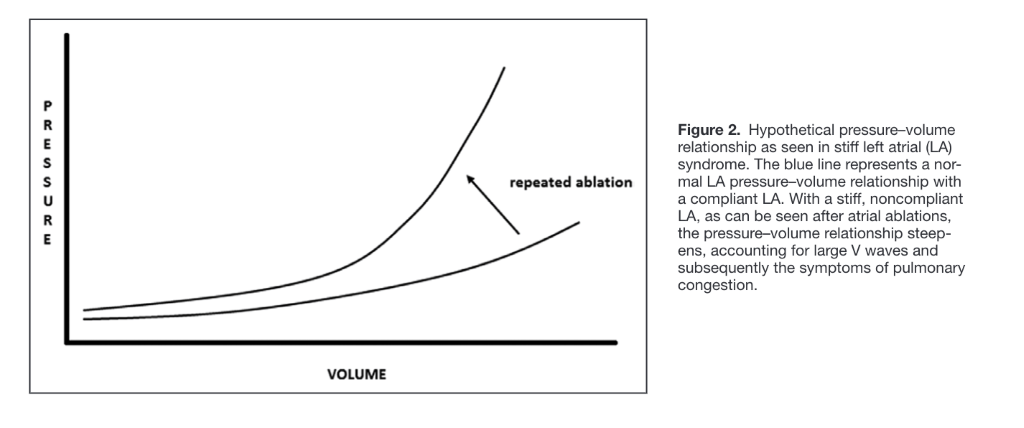
2) Which patients are at risk of developing SLAS, how is it diagnosed, and how is it managed?
- Stiff Left Atrial Syndrome was first described in the late 1980s as a complication of mitral valve surgery, and has been increasingly recognized as a complication of left atrial ablation procedures leading to atrial fibrosis. While the condition is relatively rare (occurring in ~1.4% of patients following ablation), significant heart failure symptoms and pulmonary hypertension can develop.
- While no diagnostic criteria exist, SLAS should be considered in patients with HFpEF, a small or calcified LA on imaging, and risk factors including mitral valve surgery and/or prior left atrial ablations. Invasive hemodynamics will show large v-waves in the absence of mitral regurgitation (or disproportionate to the degree of MR) and an elevated PCWP out of proportion to the LVEDP. It is important to exclude pulmonary vein stenosis, another potential complication of ablation.
- Management consists primarily of diuretics and reducing ventricular afterload as tolerated, though an intra-atrial septostomy could be considered in refractory cases.
- Notably, SLAS may be asymptomatic in many patients due to the compliance of the pulmonary venous vascular system, which can store blood volume without significant increases in pressure. However, this compliance could become overwhelmed in certain stressed states or exercise.
3) Our patient experienced a stressor in the form of high output heart failure; what is the pathophysiology of high output heart failure, and what is your differential for high output heart failure?
- While a number of causes for high output heart failure exist, they share an underlying pathophysiology of excessively decreased systemic vascular resistance and increased metabolic demand. The persistently low SVR leads to decreased ventricular afterload, increased LV emptying and thus increased stroke volume and cardiac output. This subsequently leads to increased preload and symptoms of congestive heart failure. Furthermore, increased oxygen demands requires increased cardiac output. Additionally, the persistently low SVR causes low renal perfusion pressure (renal hypoperfusion) which leads to RAAS activation and volume expansion
- Diagnosis is based on echocardiographic evaluation, RHC hemodynamics, and an identified cause of a high output state. TTE may show normal or reduced ejection fraction; additional findings may include a dilated IVC, RV enlargement or dysfunction, elevated estimated pulmonary artery pressures, and/or LV enlargement. RHC typically shows a CO > 8 L/min or a CI > 4 L/min/m2, though these cutoffs are not absolute.
- The differential for high output heart failure includes etiologies secondary to predominantly low SVR (e.g., obesity, cirrhosis, AV fistula) versus those secondary to increased metabolic drive (e.g., hyperthyroidism, myeloproliferative disorders). See the CNCR episode from the Johns Hopkins Hospital for more details!
4) How does thiamine deficiency lead to high output heart failure?
- Thiamine is vital to aerobic metabolism in the Krebs cycle and the Pentose Phosphate Pathway. In states of thiamine deficiency, anaerobic metabolism is favored over aerobic metabolism, leading to increased levels of lactate and pyruvate. This leads to a decrease in adenosine triphosphate (ATP) and increase in adenosine monophosphate (AMP), which is released into skeletal muscle as adenosine. This release of adenosine leads to vasodilation and decreased systemic vascular resistance through shunt physiology.
- Arterial hypoperfusion of the kidneys leads to activation of the RAAS and expansion of plasma volume. Increased oxygen demand lead to an increased cardiac output.
- Importantly, CO by thermodilution and Fick may be discrepant in Beriberi! This is because mitochondria are unable to utilize O2 by performing aerobic metabolism. Thus, less oxygen is extracted from the blood, and venous oxygen saturations will be relatively elevated. This may leads to an erroneously elevated CO by Fick’s method as compared to thermodilution!
5) Lets bring it all together! Cardionerds, what is your illness script for Beriberi?
- Pathophysiology: As detailed above, thiamine deficiency causes an increase in anaerobic metabolism, increased oxygen demand and systemic vasodilation through increased adenosine levels.
- Epidemiology: Patient populations at risk for severe thiamine deficiency include patients with severe malnutrition, chronic alcohol use, incarceration, social isolation, refugee populations, history of bariatric surgery, or chronic loop diuretic use. Notably, 90% of patients on diuretics can develop some level of thiamine deficiency.
- Signs/Symptoms: “Dry” beriberi involves symmetrical peripheral neuropathy, primarily in the distal extremities. “Wet” beriberi is characterized by high output heart failure and can lead to shock in severe cases.
- Diagnosis: Thiamine deficiency is difficult to diagnose. Blood thiamine levels can be low in acute illness and do not reflect total body stores. Erythrocyte transketolase activity and thiamine pyrophosphate effect tests can be used, though these tests have poor specificity and sensitivity. The gold standard is high performance liquid chromatography, though access to this test is expensive and not commonly available.
- As a historical note, in 1945, Marion Blankenford developed diagnostic criteria for wet beriberi, which includes evidence of an enlarged heart with normal rhythm, dependent edema, elevated venous pressure, peripheral neuritis or pellagra, nonspecific alternans on ECG, no evidence of other cardiac disease, at least 3 months of thiamine deficiency, and improvement in symptoms and reduction in heart size following thiamine replacement.
- Treatment: The cornerstone of wet beriberi management is supportive treatment of heart failure while replacing thiamine stores. A rapid and dramatic improvement following thiamine replacement is diagnostic of wet beriberi.
References
- Bisbal, F., Baranchuk, A., Braunwald, E., et al. (2020). Atrial Failure as a Clinical Entity: JACC Review Topic of the Week. Journal of the American College of Cardiology, 75(2), 222–232.
- Gibson, D. N., Di Biase, L., Mohanty, P., Patel, J. D., Bai, R., Sanchez, J., Burkhardt, J. D., Heywood, J. T., Johnson, A. D., Rubenson, D. S., Horton, R., et al. (2011). Stiff left atrial syndrome after catheter ablation for atrial fibrillation: clinical characterization, prevalence, and predictors. Heart rhythm, 8(9), 1364–1371.
- Maeder, M. T., Nägele, R., Rohner, P., & Weilenmann, D. (2018). Pulmonary hypertension in stiff left atrial syndrome: pathogenesis and treatment in one. ESC heart failure, 5(1), 189–192.
- Urey, M. A., Darden, D., Stoller, D., et al. (2017). Stiff Left Atrial Syndrome After Multiple Percutaneous Catheter Ablations: Role for Invasive Hemodynamic Exercise Testing. Circulation. Heart failure, 10(5), e003885.
- Durstenfeld, M. S., & Hsue, P. Y. (2020). An Unusual, Reversible Cause of Acute High-Output Heart Failure Complicated by Refractory Shock. Circulation, 142(9), 901–905.
- Reddy, Y., Melenovsky, V., Redfield, M. M., Nishimura, R. A., et al. (2016). High-Output Heart Failure: A 15-Year Experience. Journal of the American College of Cardiology, 68(5), 473–482.




















