
CardioNerds Journal Club is a monthly forum for CardioNerds to discuss and breakdown recent publications on twitter and are produced with a corresponding infographic and detailed blog post. For more information, check out the CardioNerds Journal Club Page. This Journal Club focuses on the SODIUM-HF Trial.

Table of contents for the SODIUM-HF Trial summary:



June 30, 2022
Reduction of dietary sodium to less than 100 mmol in heart failure (SODIUM-HF): an international, open-label, randomized, controlled trial.
Ezekowitz JA, Colin-Ramirez E, Ross H, Escobedo J, Macdonald P, Troughton R, Saldarriaga C, Wendimagegn A, McAlister, F, Arcand J, Atheron J,Doughty R, Gupta M, Howlett J, Jaffer S, Lavoie A, Lund M, Marwick T, McKelvie R, Moe G, Pandey AS, Porepa L, Rajda M, Rheault H, Singh J, Toma M, Virani S, Zieroth S, on behalf of the SODIUM-HF investigators.
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(22)00369-5/fulltext
https://read.qxmd.com/read/35381194/

Relevant Literature – SODIUM-HF Trial

Relevant Guidelines – SODIUM-HF Trial
- The AHA currently recommends reducing sodium intake to <2300 mg/d for general cardiovascular health promotion; however, there are no trials to support this level of restriction in patients with HF.
- The 2022 AHA/ACC/HSFA Heart Failure Guidelines recommend patients with heart failure stage C avoid excess sodium intake to reduce congestive symptoms.

Study Rationale – SODIUM-HF Trial
There is a lack of quality data regarding the clinical benefits or harm of sodium restriction in patients with HF. Most studies analyzing sodium restrictions on HF patients are small samples without sufficient racial and ethnic diversity. There is also a lack of data surrounding the correct threshold of sodium restriction for clinical benefit and uncertainty about which subgroups benefit most from sodium restriction.
Objective
The goal of this trial was to evaluate if a low-sodium diet (<1500 mg daily) compared to usual care in ambulatory patients with heart failure would prevent adverse cardiovascular events.

Trial
International, open-label, randomized, controlled trial conducted across 26 sites in six countries (Australia, Canada, Chile, Colombia, Mexico, and New Zealand).
Intervention
Patients were randomly assigned (1:1), using a standard number generator and varying block sizes of two, four, or six, stratified by site, to either usual care according to local guidelines or a low sodium diet of less than 100 mmol (i.e., <1500 mg/day).
Enrollment Criteria
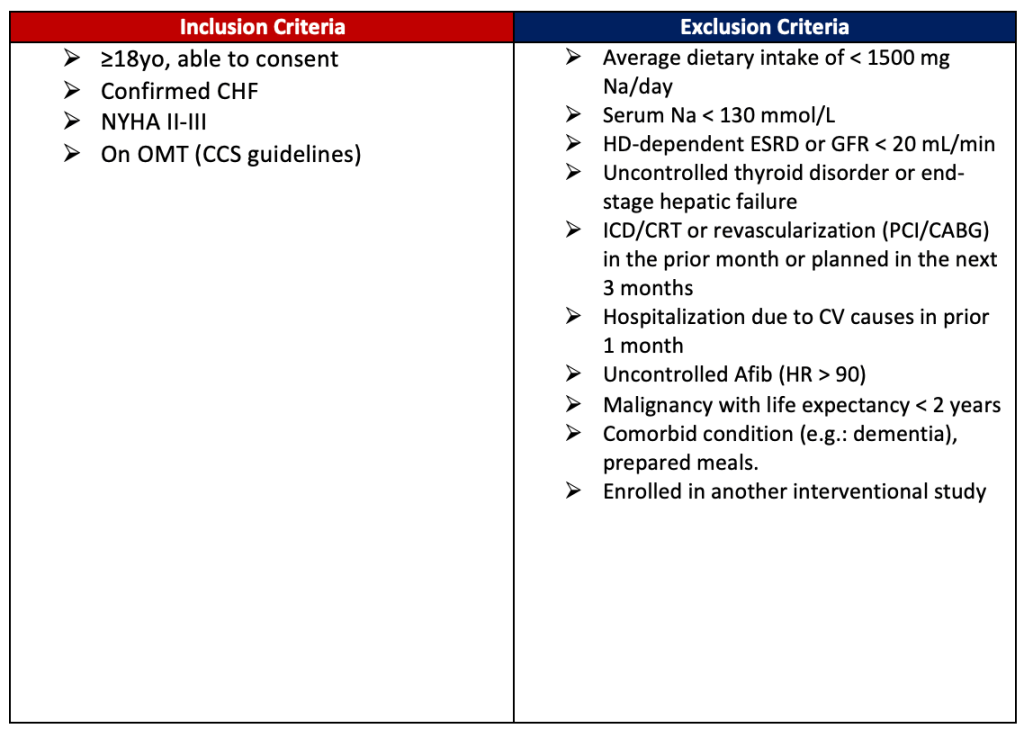
Outcomes

Statistical Analysis
- Sample size: The final sample size was approved by the data and safety monitoring board with 2404 participants (1202 in each group), sufficient to detect a relative reduction of 33% in the incidence of primary-composite outcome (assuming baseline incidence rate of primary outcome was 16% with 10% non-adherence and 5% lost to follow-up) with 85% power and a two-sided alpha of 0.05. Blinded reassessment of sample size after enrollment of 800 patients showed a primary-outcome incidence of 30%, confirming that the above number of participants would be sufficient.
- Analysis: Intention-to-treat model. For cases where the primary-outcome was undetermined, primary analysis used multiple imputations for primary outcome. Log-binomial regression was used to generate adjusted relative risk and 95% confidence interval for primary outcomes.

Sample size: 992 patients
Participant Characteristics:
- Median age was 67 years
- 66% were men and 33% were women
- Data on race and ethnicity were not collected
- 68% had heart failure for at least 1 year before enrollment
- 33% had been admitted to the hospital due to heart failure in the past 12 months
- Median EF was 36%

- In the 325 patients with a natriuretic peptide measurement in the 90 days before enrolment, median BNP was 197 pg/mL and median NT-proBNP was 801 pg/mL
- 81% were receiving an angiotensin-converting enzyme inhibitor, an angiotensin receptor blocker, or sacubitril–valsartan
- 87% were receiving a β blocker
- 57% were receiving a mineralocorticoid receptor antagonist

Outcomes
Primary Outcomes:
- Within 12 months, the primary outcome (cardiovascular-related hospitalization, cardiovascular-related emergency department visit, or all-cause death) had occurred in 60 (15%) of 397 patients in the low sodium diet group and 70 (17%) of 409 in the usual care group (HR 0.89 [95% CI 0.63–1.26]; p = 0.53).
Secondary Outcomes:
- Cardiovascular-related hospitalization occurred in 40 (10%) patients in the low sodium diet group and 51 (12%) patients in the usual care group (HR 0.82 [0.54–1.24]; p = 0.36)
- Cardiovascular-related emergency department visits occurred in 17 (4%) patients in the low sodium diet group and 15 (4%) patients in the usual care group (HR 1.21 [0.60–2.41]; p = 0.60)
- All-cause death occurred in 22 (6%) patients in the low sodium diet group and 17 (4%) in the usual care group (HR 1.38 [0.73–2.60]; p=0.32).


No difference in 6-min walk distance at 12 months between the low sodium diet group and the usual care group (Figure C).

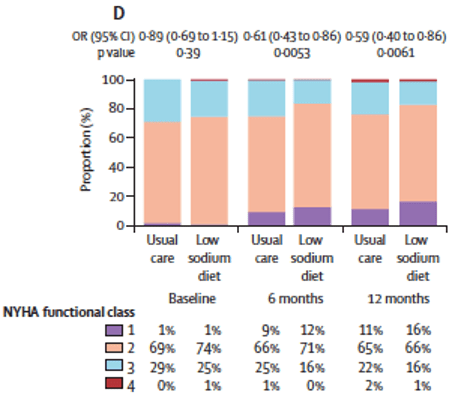
- There was a significant difference between groups in NYHA functional class at 12 months, with the low sodium diet group having a greater likelihood of improving by one NYHA class than the usual care group (odds ratio 0.59 [95% CI 0.40–0.86]; p=0.0061 (Figure D)
- Post-Hoc Sensitivity analysis found no significant interaction between the primary outcome and baseline dietary sodium intake (≤1500, 1501–3000, and >3000 mg/day; p interaction = 0.63)
Adverse Events:
No safety events attributable to the trial were reported in either the low sodium diet or usual care groups.

Conclusions
In ambulatory patients with heart failure, a dietary intervention to reduce sodium intake did not reduce clinical events; however, there was a small improvement in quality of life and NYHA functional class.
Limitations & Considerations
- Patients were not masked to study group assignment, which could be a potential source of bias, especially for the secondary outcomes, including NYHA functional class, KCCQ, and 6-min walking distance.
- Contamination bias could have occurred as some patients may have reduced their sodium intake in the usual care group; however, this was not evident.
- Data on urinary or other biomarkers were not collected secondary to the lack of resources available for the trial.
- The trial was stopped early and so might overestimate the efficacy (or risk) of an intervention.
- The inclusion criteria did not require NT-proBNP but instead relied on clinical diagnoses at sites familiar with the care of patients with heart failure likely resulting in a mixture of higher and lower risk patients enrolled in the trial. Nevertheless, the event rate in SODIUM-HF was similar to that of other trials of ambulatory patients with heart failure.


The published archive features curated twitter highlights from the journal club event.

SUMMARY:
Dr. Breanna Hansen, Internal Medicine Resident, Cedars Sinai
Dr. Zaid Safiullah, Internal Medicine Resident, Johns Hopkins Hospital
VISUAL ABSTRACT:
Dr. Ashish Correa, Heart Failure Fellow, Mount Sinai Heart
JOURNAL CLUB PROMO GRAPHIC:
Dr. Christian Faaborg-Andersen, Internal Medicine Resident, Mass General Hospital
TWEET PREPARATION:
Dr. Aravind Kalluri, Internal Medicine Resident at University of Pennsylvania
Dr. Alaa Diab, Internal Medicine Resident, Greater Baltimore Medical Center
HOUSE JONES CHIEF FELLOW:
Dr. Patrick Zakka, Cardiology Fellow, UCLA
DIRECTOR of JOURNAL CLUB:
Dr. Devesh Rai, @DeveshRaiMD, Cardiology fellow at Rochester General Hospital





